Designed for scalable hydrogen production
Our solid oxide electrolysis (SOEC) technology is built around a plug-and-play modular architecture that scales with your production needs — whether you start with a single 8-core section or multi-megawatt setup. This flexibility lowers project risk, shortens deployment time, and keeps costs under control.
Designed with non-noble materials to reduce supply chain risk and fully optimised for downstream integration, SOEC ensures maximum uptime and long-term performance.
Click to explore the design that makes it all work — from cell to stack, core to section, and right through to full-scale production.
.png)








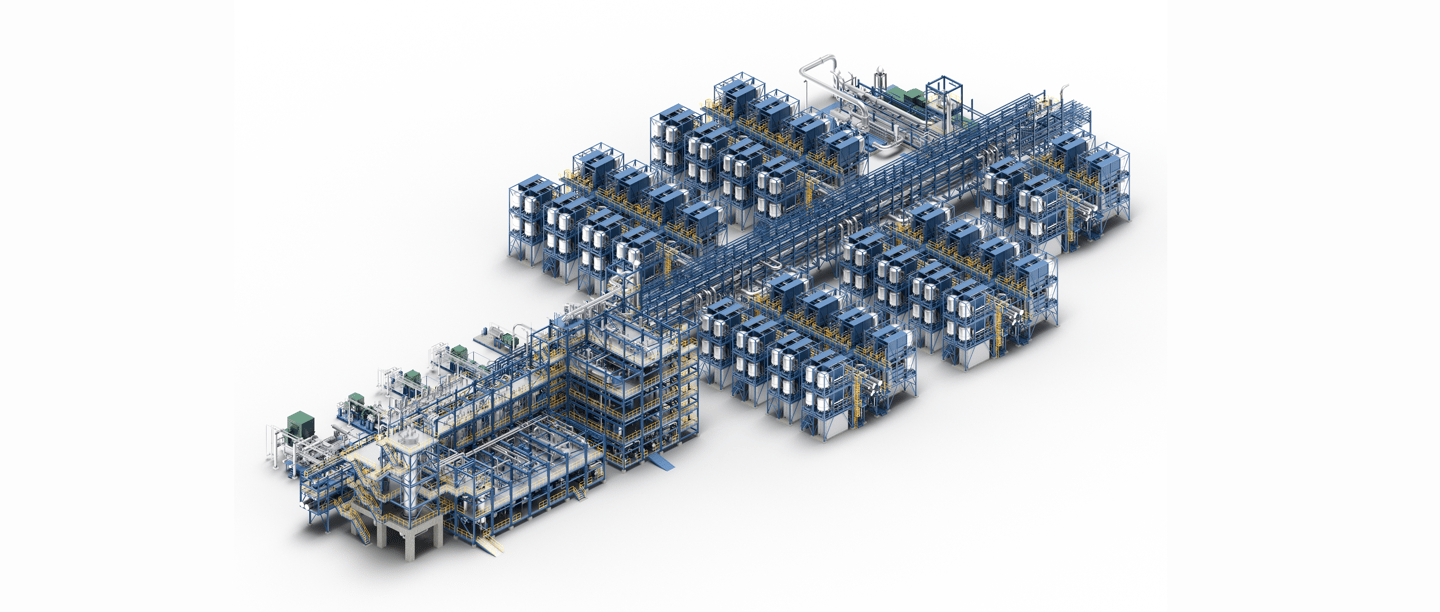



.png?width=616&height=560&name=SOEC_Sektion%20(1).png)